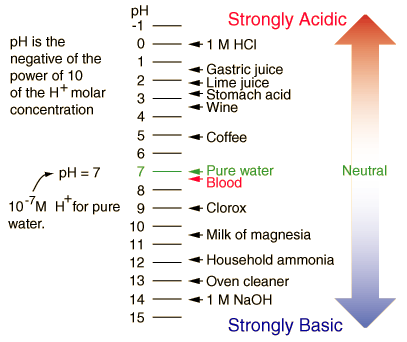
Understanding the pH Scale
- pH stands for (presence of Hydrogen).
- Numbered from 0 to 14.
- The lower the pH number - the higher Acid.
- That means more Hydrogen Ions (H+).
- The higher the pH - the higher the Base.
- That means less Hydrogen Ions (H+).
pH : Definition and measurement units
- pH is a measure of how acidic/basic water is.
- The range goes from 0 - 14, with 7 being neutral.
- pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base.
- pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water.
- Water that has more free hydrogen ions is acidic, whereas water that has more free hydroxyl ions is basic.
- Since pH can be affected by chemicals in the water, pH is an important indicator of water that is changing chemically.
- pH is reported in "logarithmic units".
- Each number represents a 10-fold change in the acidity/basicness of the water.
- Water with a pH of 5 is fifty times more acidic than water having a pH of 6.
Help Me !
# - At First you have to select one solution which you need
# - Use the Upper button (circle) to drop the solution into the Flask
# - Use the Bottom button to decrease the solution level
# - If we mix the solution with water(neutral) there will be a color change in the flask,
at the same time you can see the PH changes in the PH scalar.
Report a Problem
Please copy and paste the information below into an email to
chemixvisualize@gmail.com,
and then fill in the blanks.
Test device:
Operating System:
Browser:
Problem description:
Steps to reproduce:
Screenshots:
Chemix Visualize
Version : 1.1.1
Copyright © <2016> MozillaSciencelab-Chemix Contributors
Development Team
Mr.R.RatheeswaranMs.T.Suganya
Ms.K.Kopitha
Ms.A.Ajantha
(University Of Jaffna.)
Thanks
Lecturer Mr.K.Sarveswaran (RAD Project (2013-2014) Guider)MozillaSciencelab - Chemix Team
Terms,Privacy and Licensing